
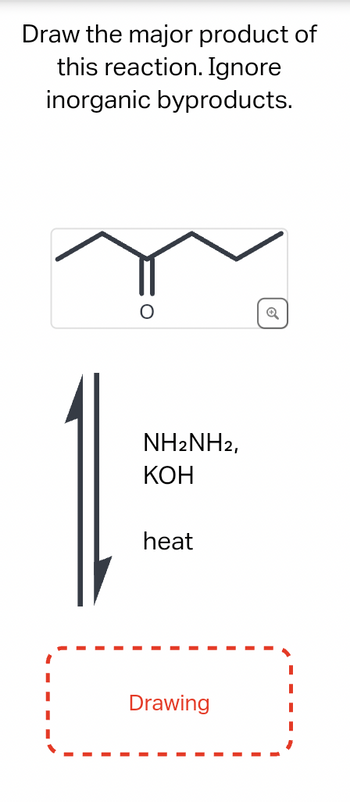
SOLVED: NH2NH2 NIM = Two products which contain carbon H2O 1. Heat H2O OH Hint: Two things happen, the mass goes down 44 g/mol and then 18 g/mol OH

Development and Scale-Up of a Continuous Manufacturing Process for a Hydrazine Condensation Reaction | Organic Process Research & Development

SOLVED: Reagents, conditions, and products as appropriate: A 1 NH2NH2 cat. HCl, -H2O CH3CH2NH2 cat. HCl, -H2O 2) KOH, heat 2 (2 pts) Imines hydrolyze in acidic aqueous media to form ketones

Synthesis of the target compounds 24-43. Reagents and conditions: (a)... | Download Scientific Diagram
![Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf](https://www.ncbi.nlm.nih.gov/books/NBK143197/bin/ml228f12.jpg)
Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf
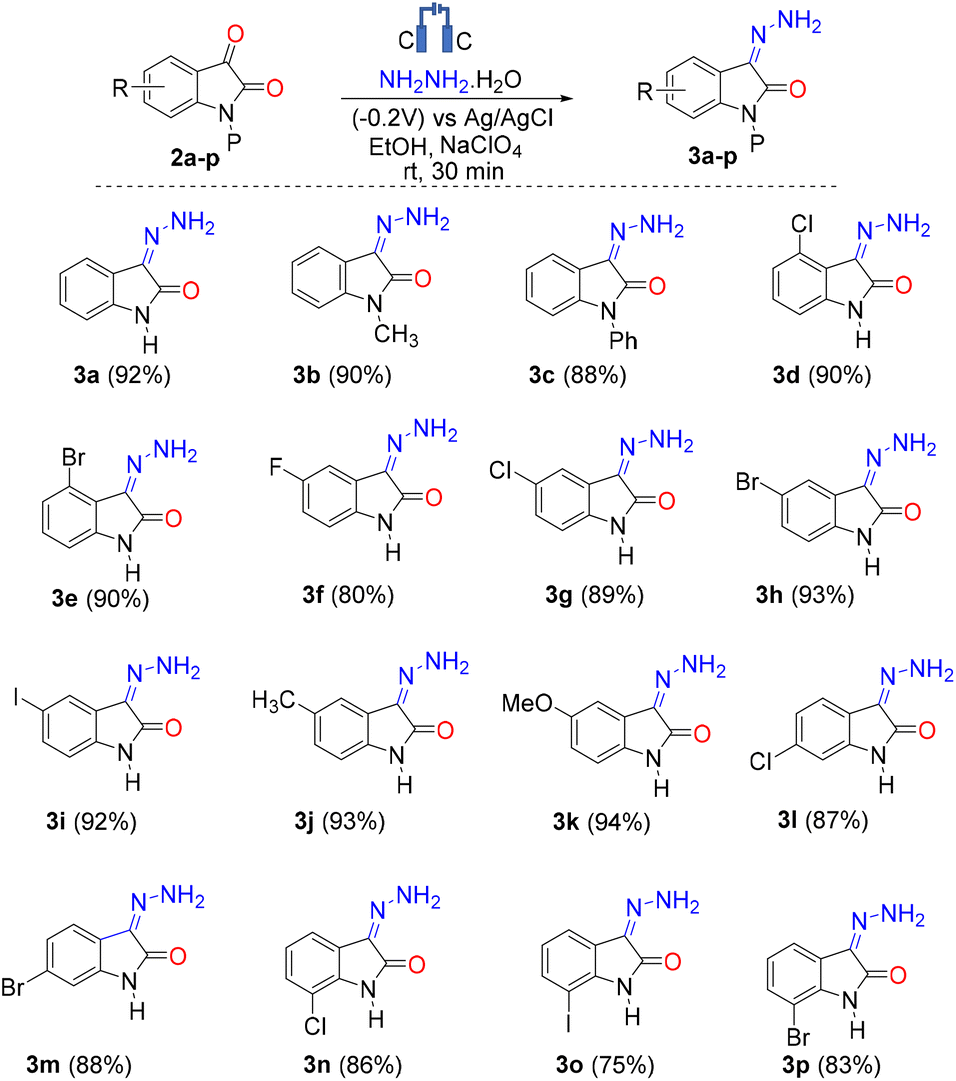
Electro-organic synthesis of isatins and hydrazones through C–N cross-coupling and C(sp 2 )–H/C(sp 3 )–H functionalization - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01128C
HYDRAZINE HYDRATE Extra Pure | Laboratory chemical suppliers, Laboratory Chemicals, Lab chemical distributors, Laboratory chemicals manufacturer, Lab chemical supplier, Lab chemicals exporter, Lab chemical manufacturer, Alpha Chemika India.

eHydrogenation: Hydrogen‐free Electrochemical Hydrogenation - Russo - 2023 - Angewandte Chemie International Edition - Wiley Online Library

Development and Scale-Up of a Continuous Manufacturing Process for a Hydrazine Condensation Reaction | Organic Process Research & Development
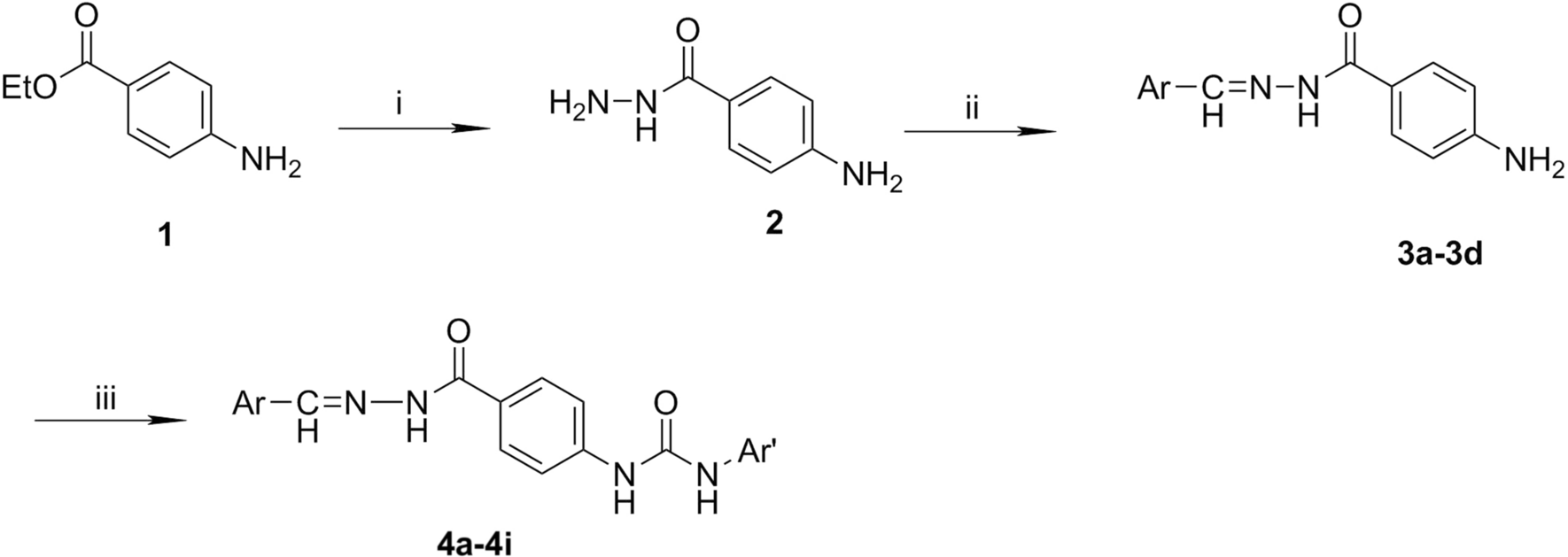
Design and synthesis of novel ureido and thioureido conjugated hydrazone derivatives with potent anticancer activity | BMC Chemistry | Full Text

Functionalized 3-hydroxy-3-aminoquinoline-oxindole hybrids as promising dual-function anti-plasmodials - ScienceDirect

Conditions and reagents: (i) NH2NH2.H2O, EtOH, reflux, 6 h; (ii) DMF,... | Download Scientific Diagram

Figure 2. Synthesis routes of sulfone derivatives containing 1,3,4-oxadiazole moiety. Reaction conditions and reagents: (a) MeOH, 98%H2SO4, reflux 5h; (b) NH2NH2▫H2O, EtOH, 25°C–reflux, 4h; (c) KOH, CS2, EtOH, 25-46-76°C, 7h; (d) NaOH,

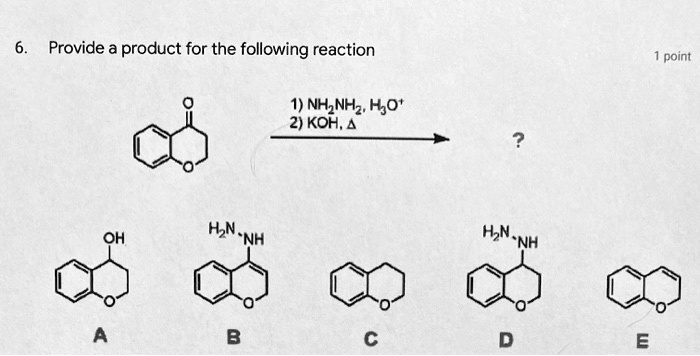




![Solved NH2NH2 H2O/HO- [heat] | Chegg.com Solved NH2NH2 H2O/HO- [heat] | Chegg.com](https://media.cheggcdn.com/media/2b8/2b86778d-b94b-43f3-ae4f-e5b21830cdfb/phpq7tc3G.png)

