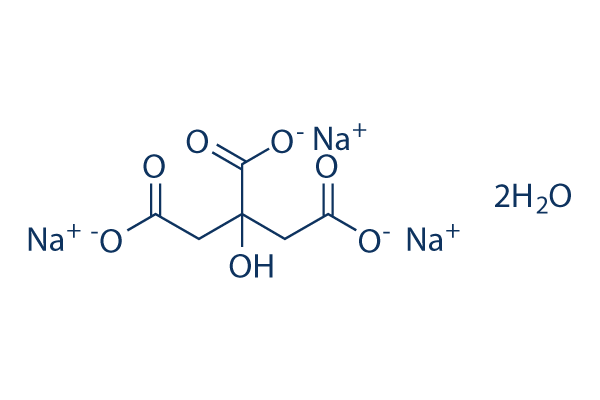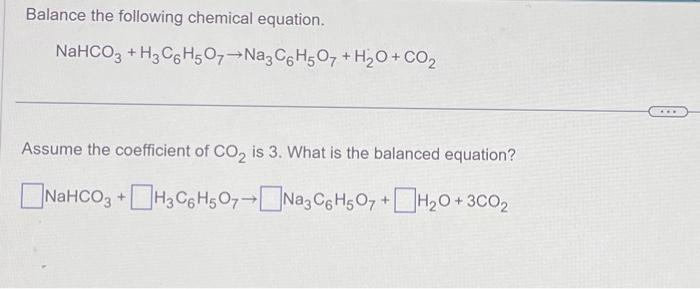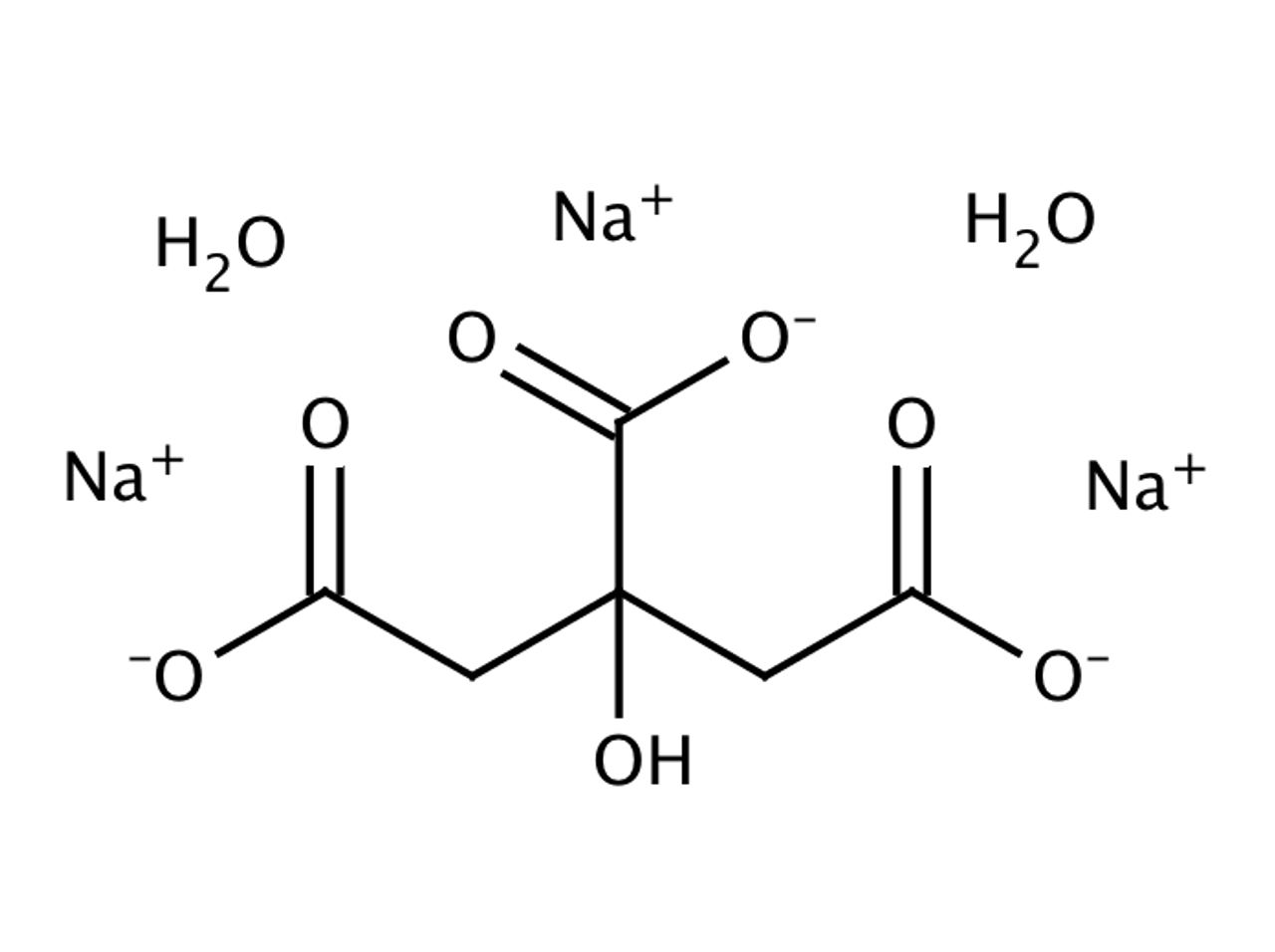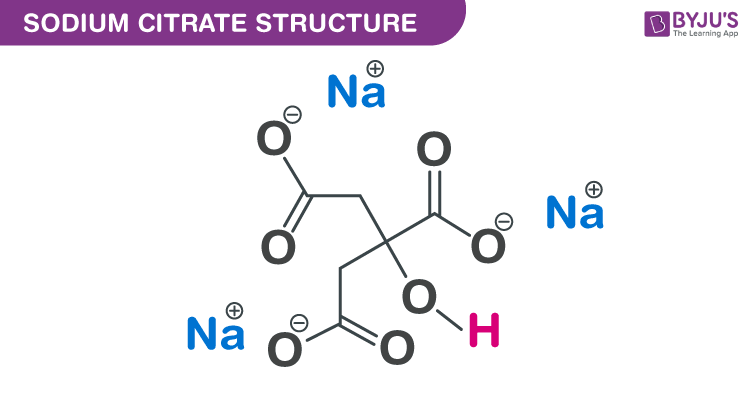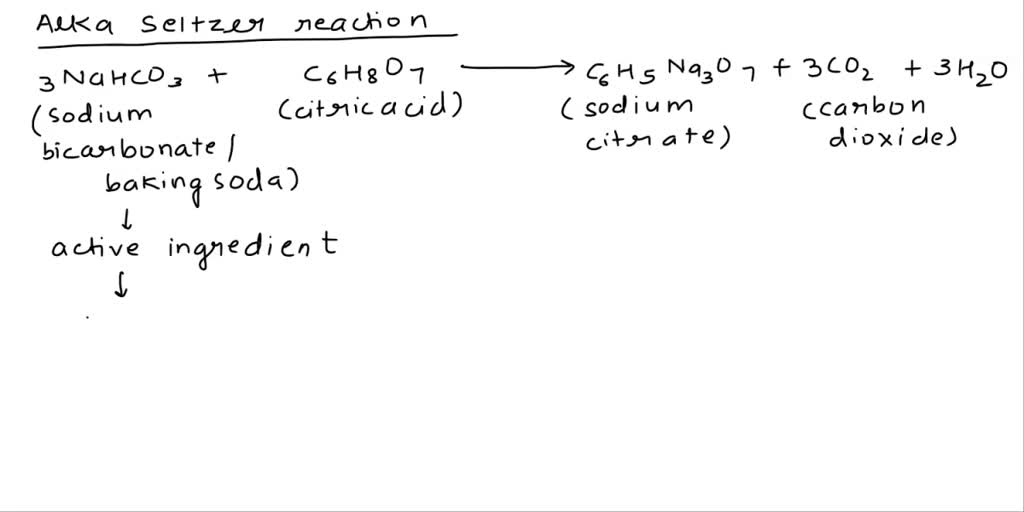
SOLVED: Classify each component of the reaction that occurs when an Alka-Seltzer tablet is dissolving in water: NaHCO3, C6H8O3, H2O, CO2, Na3C6H5O7.

How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube
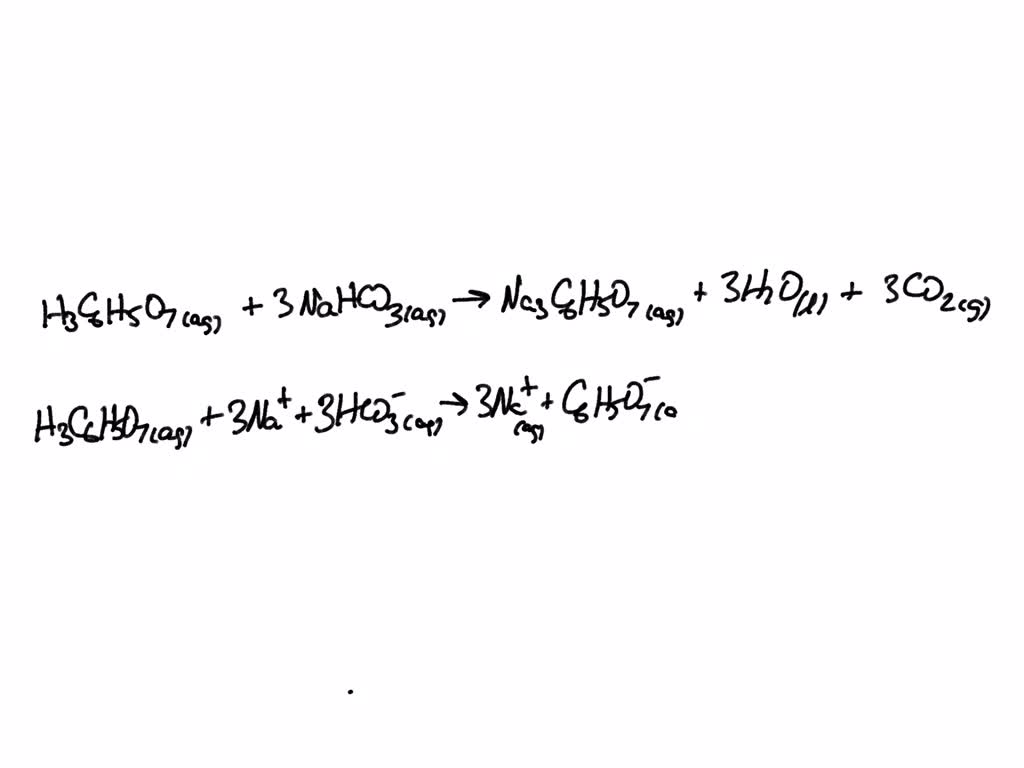
SOLVED: Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. H3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l)

How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube

SOLVED: Alka-Seltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (CO2): NaHCO3 + H3C6H5O7 ->
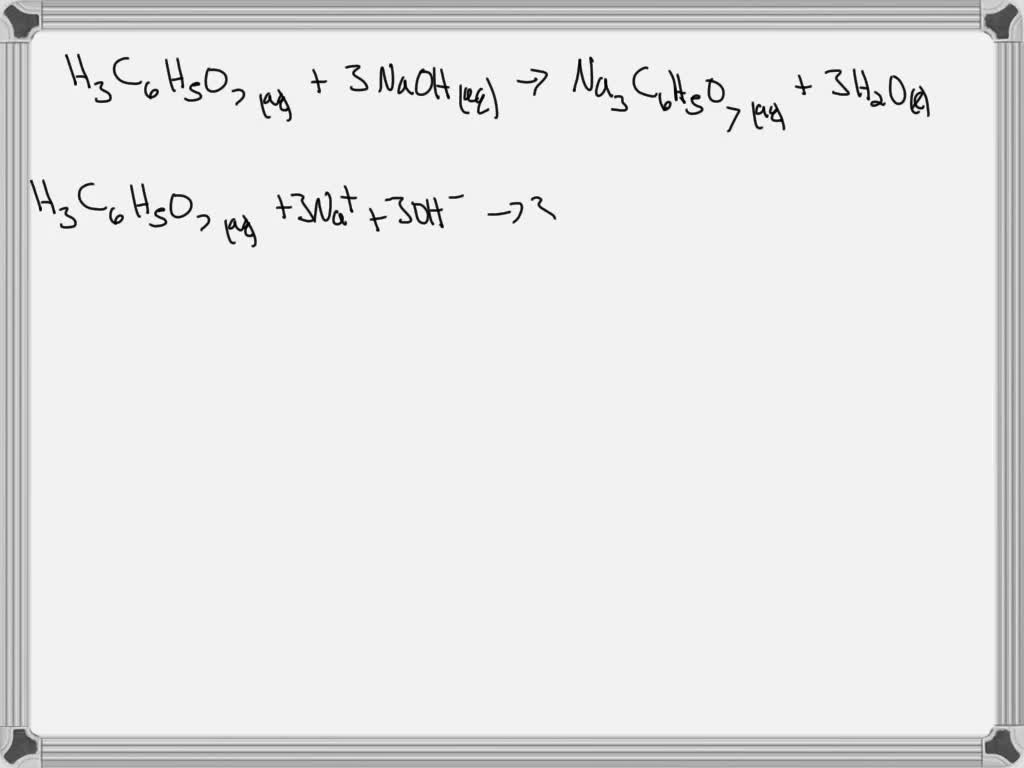
SOLVED: H3C6H5O7(aq) + 3 NaOH(aq) = Na3C6H5O7(aq) + 3 H2O(l) write complete ionic and net-ionic equations
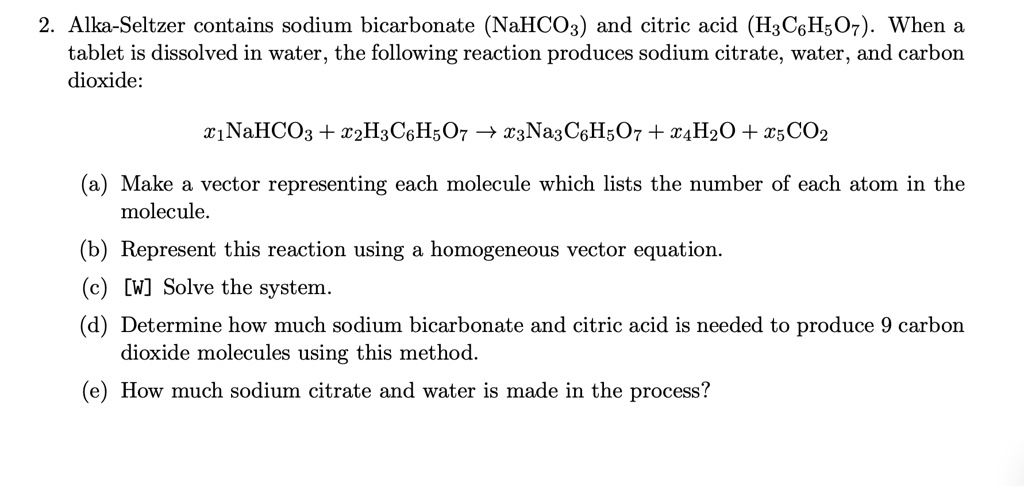
SOLVED: Alka-Seltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide: 2NaHCO3 + H3C6H5O7 â†' Na2C6H5O7 +