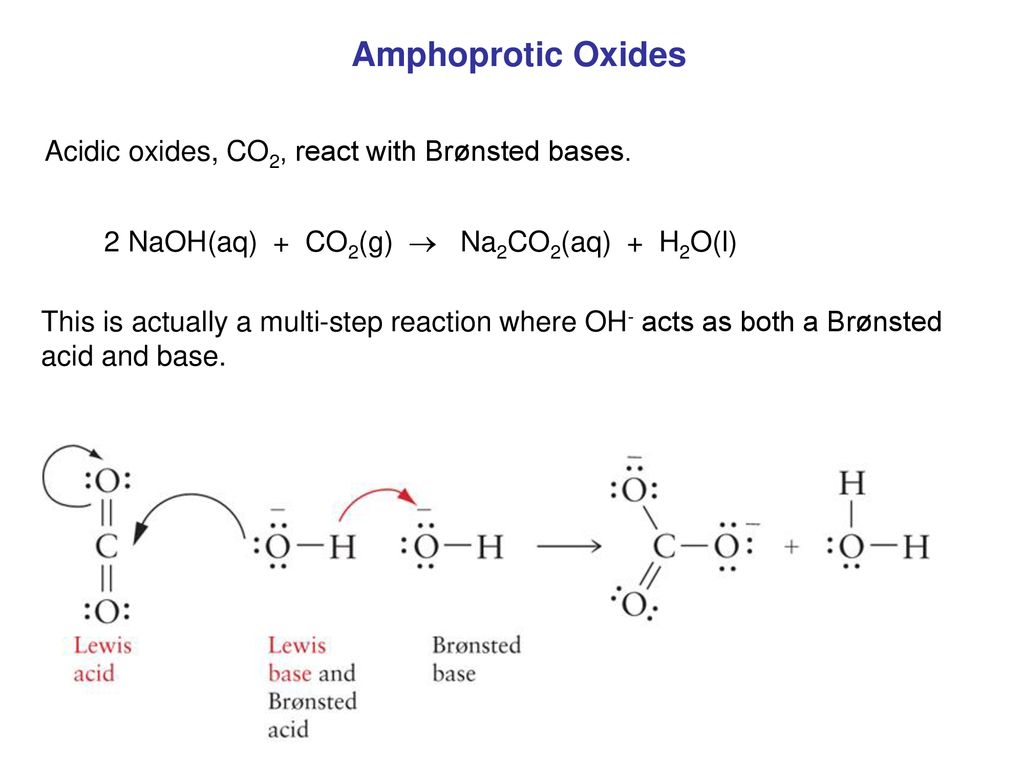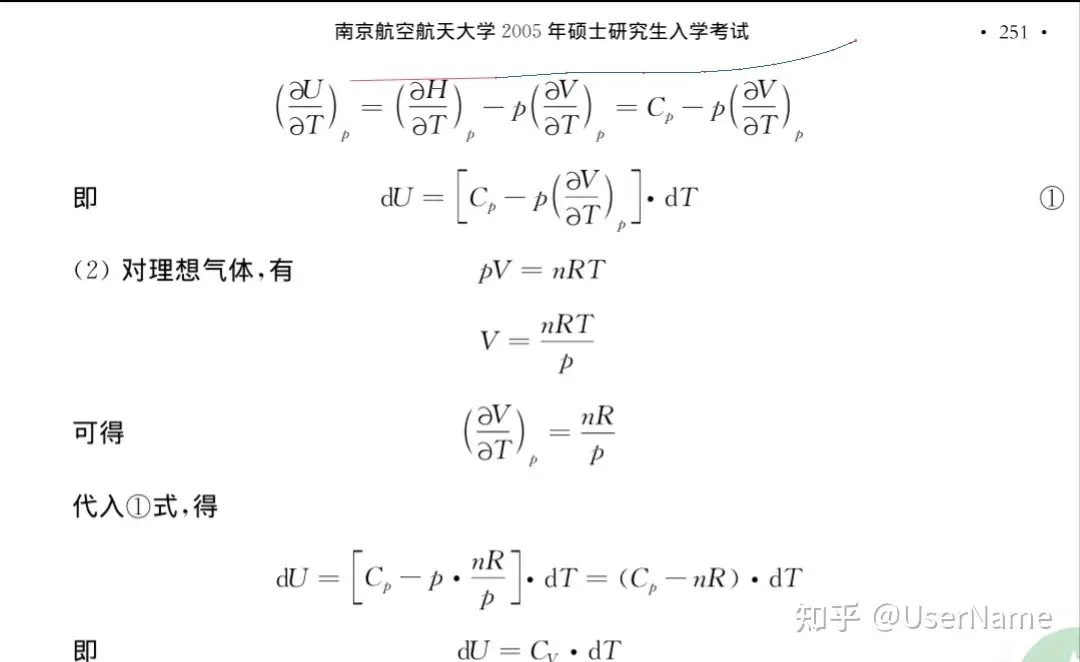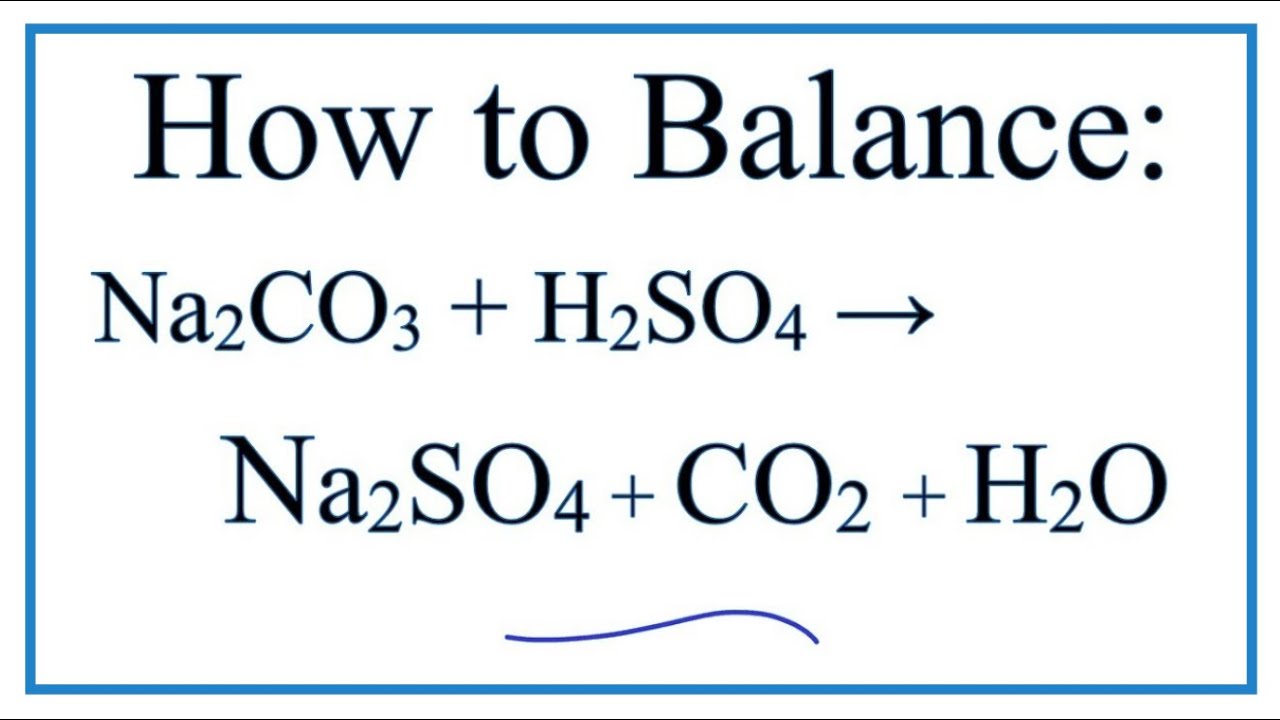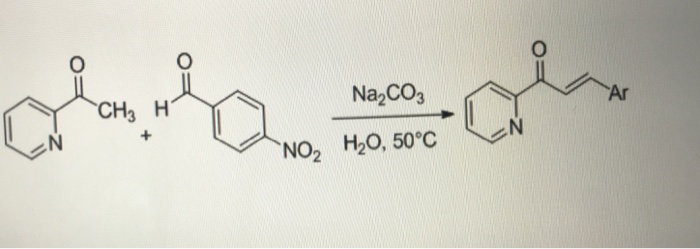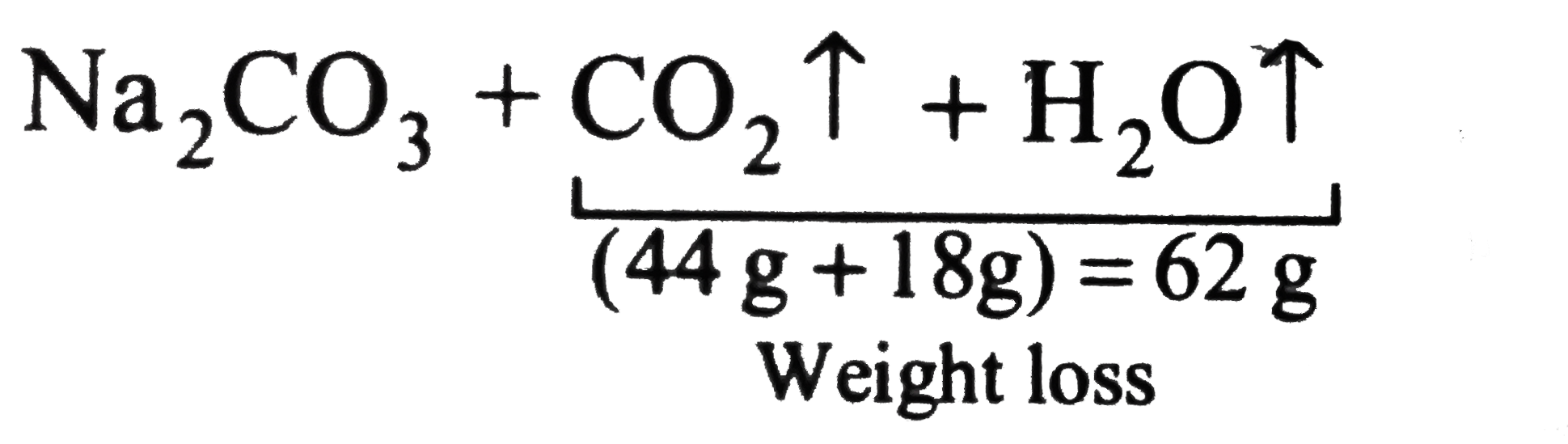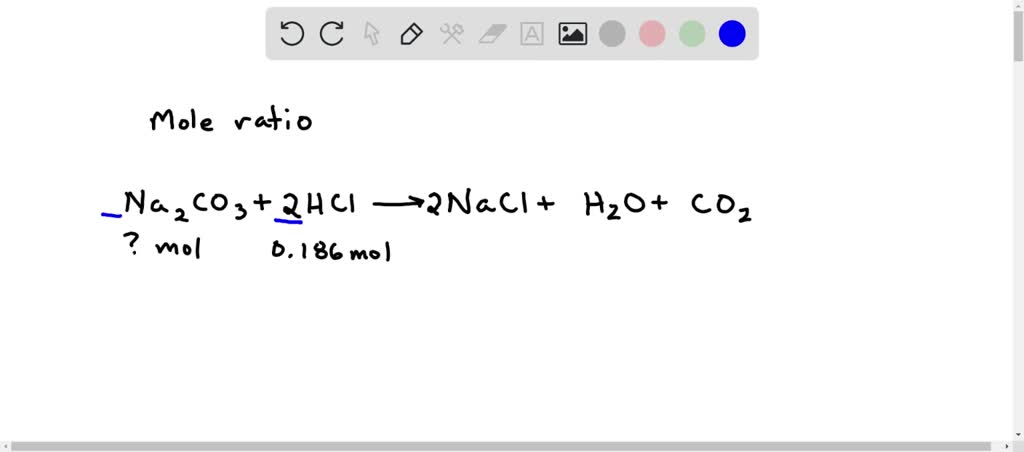
SOLVED: calculate the number of moles of sodium carbonate (Na2CO2) needed to neutralize 0,186 mole of HCI. chemical reaction : Na2CO3 + HCI —> NaCl + H20 + CO2

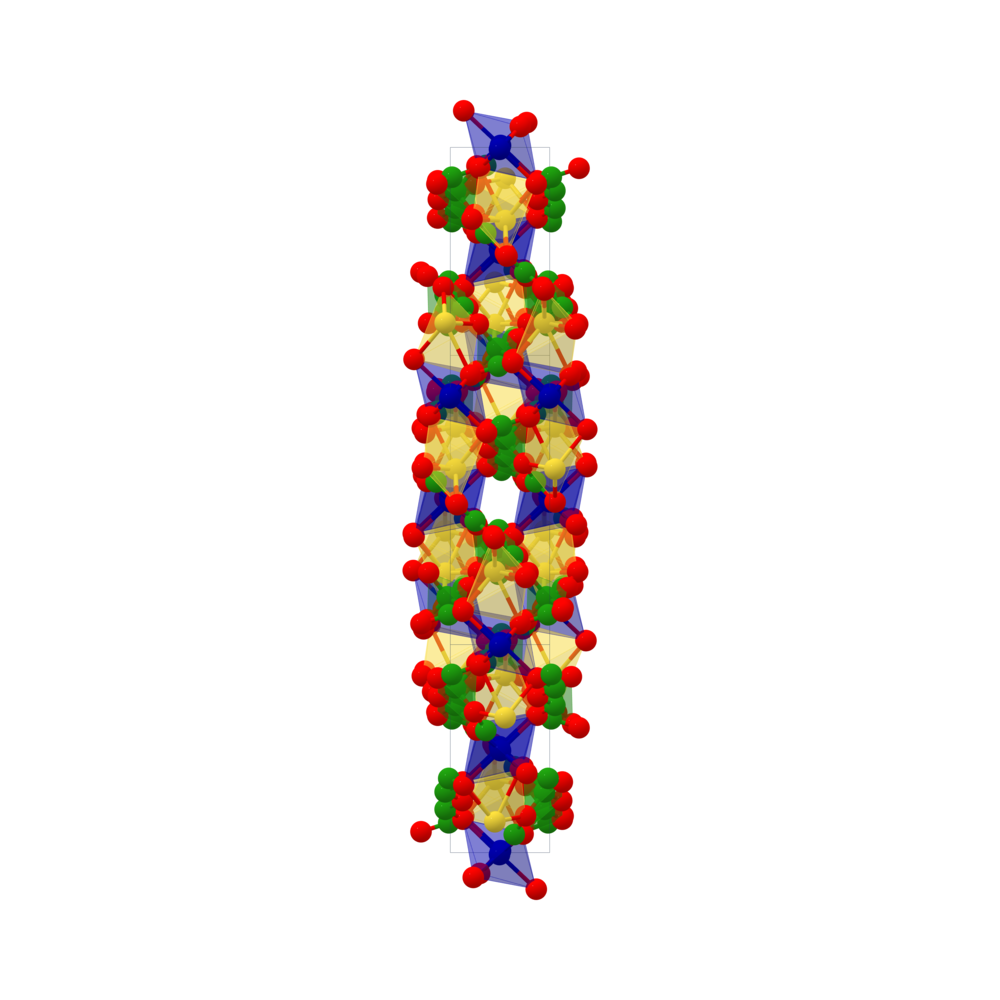
Membrane Crystallization of Sodium Carbonate for Carbon Dioxide Recovery: Effect of Impurities on the Crystal Morphology | Crystal Growth & Design

Water content diagram of the quaternary system K + // H 2 PO 4 2− , SO... | Download Scientific Diagram
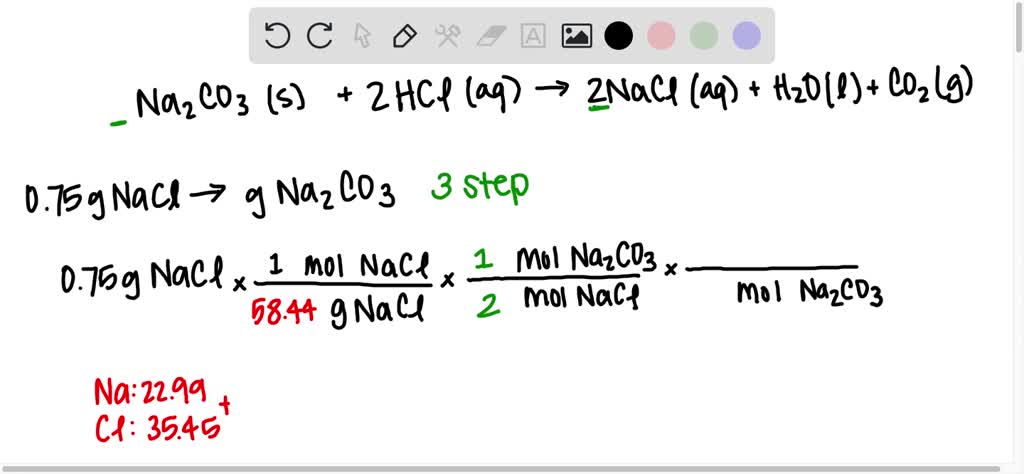
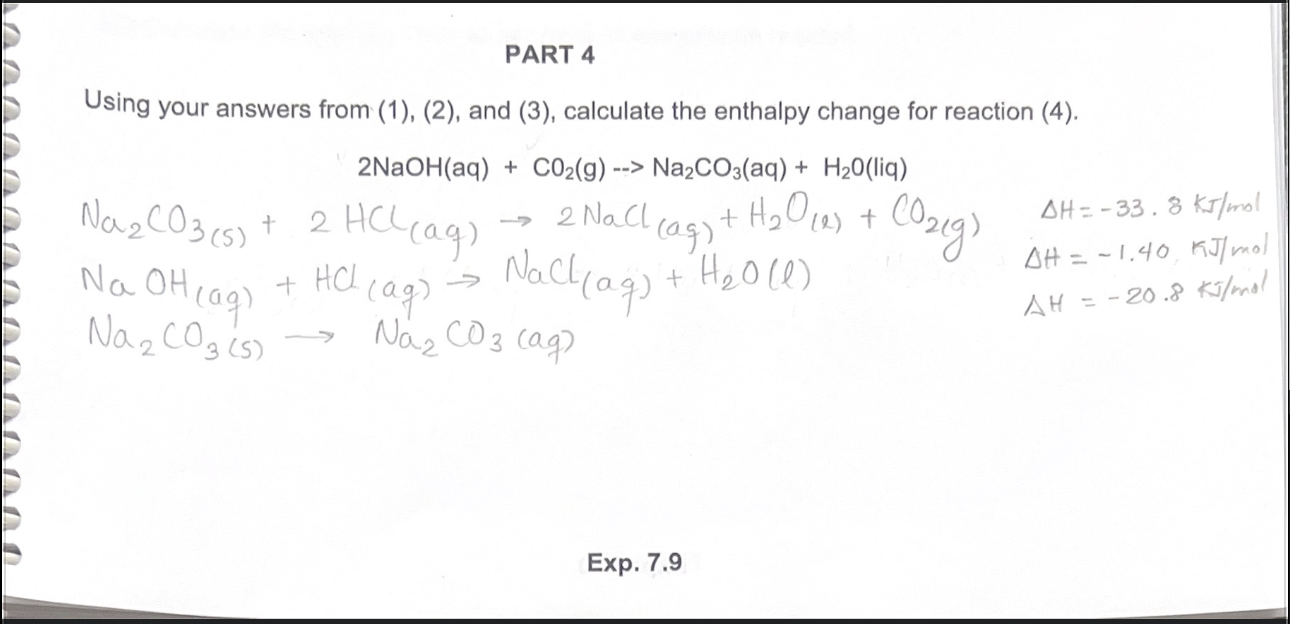
SOLVED: Na2CO3(s) + 2HCl(aq) â†' 2NaCl(aq) + H2O(l) + CO2(g) Using the balanced chemical reaction from step 1, calculate the mass of sodium carbonate (Na2CO3) that would be required to make 0.75

SOLVED: 3. Determine the mass percent of anhydrous sodium carbonate (Ns2CO1) and water in sodium carbonate decahydrate (Na2CO2-10 H2O).

Washing soda has the formula Na_(2) CO_(2).10H_(2)O. What is mass of anhydrous sodium carbonate ... - YouTube

How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Chemistry 360 | Facebook

28. State as to why: (a) Aqueous solution of Na,co, is alkaline. (b) BaO is soluble but Baso, is insoluble in water. (1) Draw structure of BeClz (vapour). (m) Complete the following:

Effect of Na2O/SiO2 and K2O/SiO2 mass ratios on the compressive strength of non-silicate metakaolin geopolymeric mortars - IOPscience