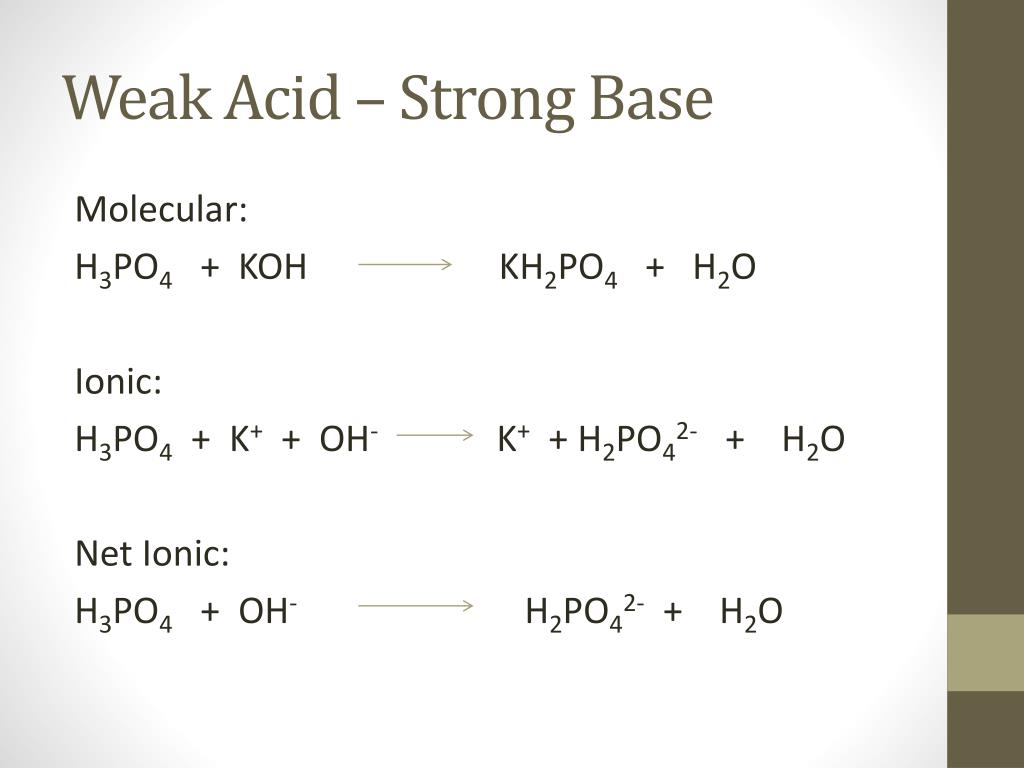
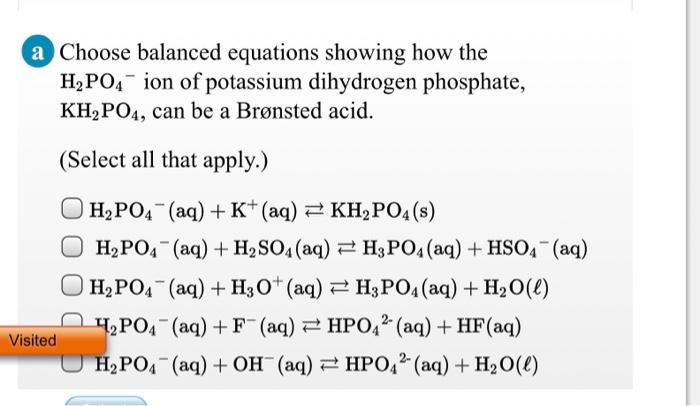
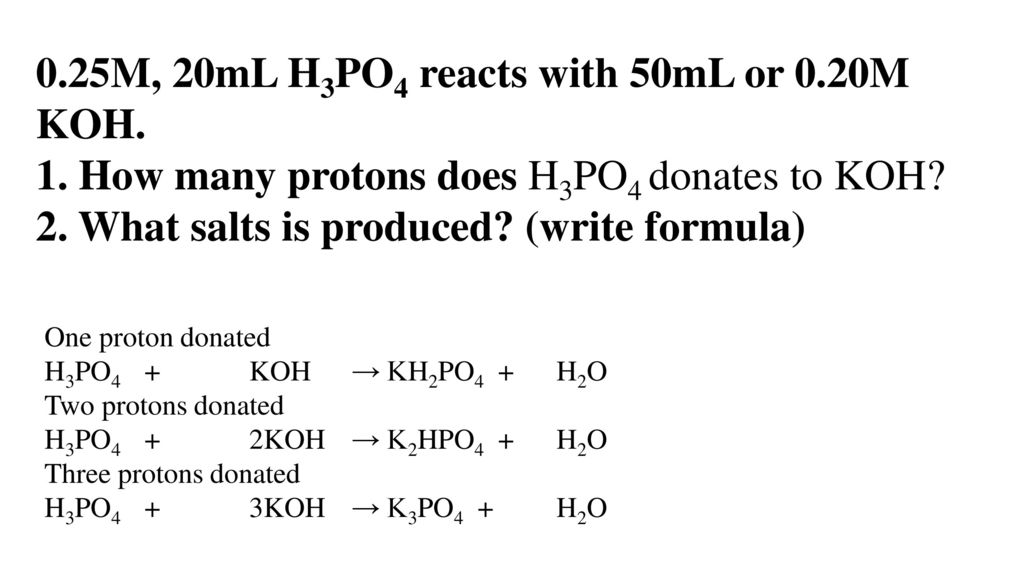
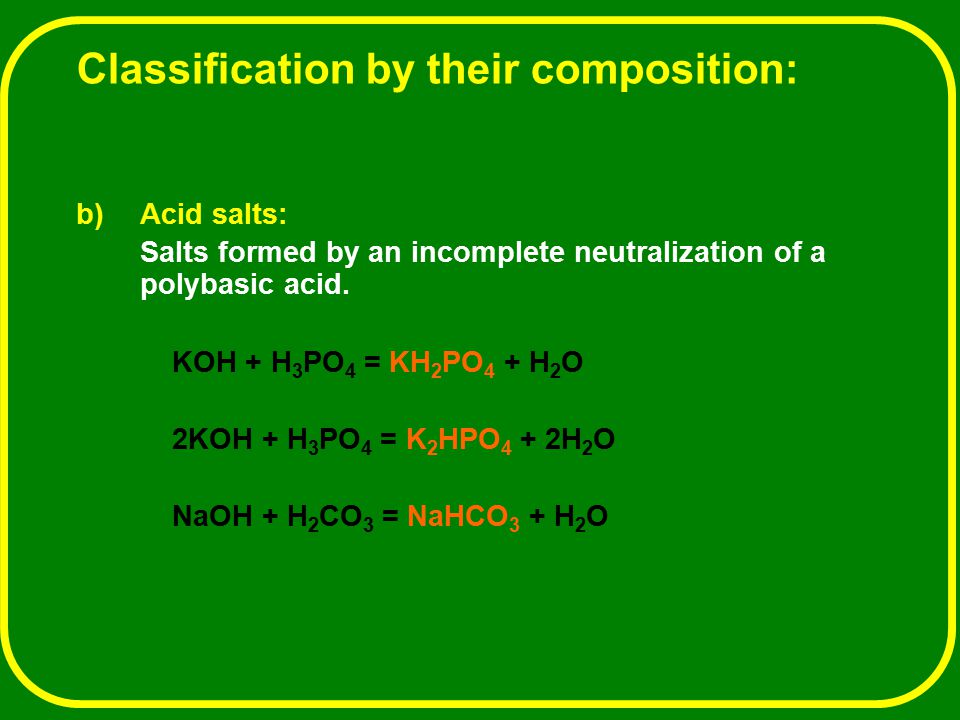
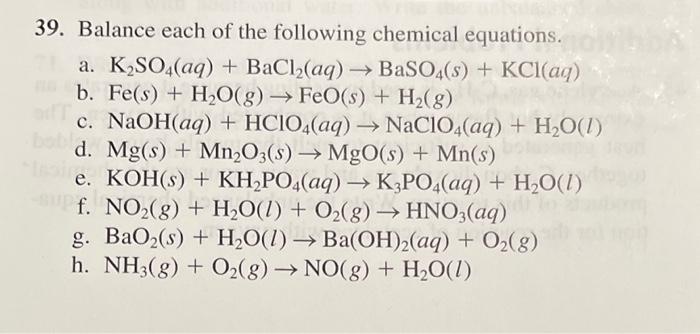OneClass: Write the chemical reaction for:KH2PO4/K2HPO4 buffer solution + NaOH (aq)andWrite the chemi...

Thermodynamic Properties Data of Ternary System KBr–KH2PO4–H2O at 298.15 K | Journal of Chemical & Engineering Data
![SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA] SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA]](https://cdn.numerade.com/ask_images/84c1fd22ef3046d0a647ee2adee981f8.jpg)
SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA]

A 0.492 g of KH_2PO_4 is titrated against a solution of 0.112 M NaOH. The volume of the base required to do this is 25.6 mL. The reaction involved is, KH_2PO_4 +
Comment calculer le pH d'une solution de KHCO3 (2M) et acide citrique (1M) (1:1) en présence d'un tampon phosphate (PBS : 10mM Na2HPO4 et KH2PO4 1,8 mM) ? - Quora

KH2PO4 crystallisation from potassium chloride and ammonium dihydrogen phosphate – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.






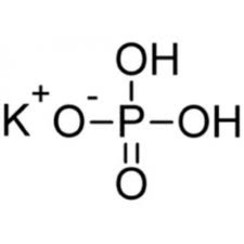

![ANSWERED] c 3 136 A 0 5 g sample of KH PO4 is titra... - Physical Chemistry - Kunduz ANSWERED] c 3 136 A 0 5 g sample of KH PO4 is titra... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20200703122613132777-1655732.jpg)