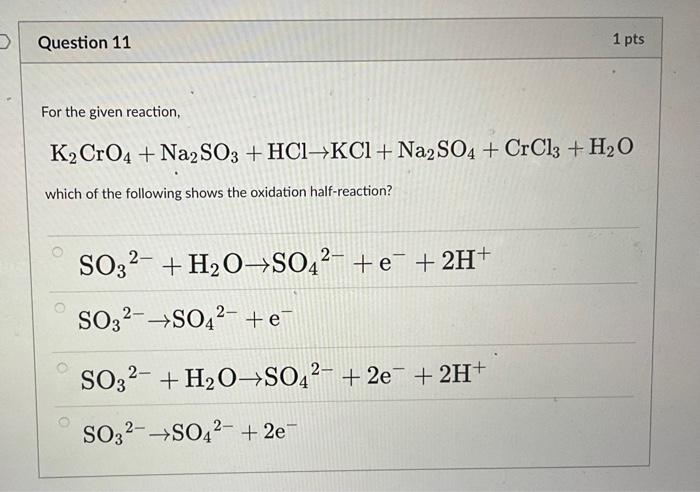
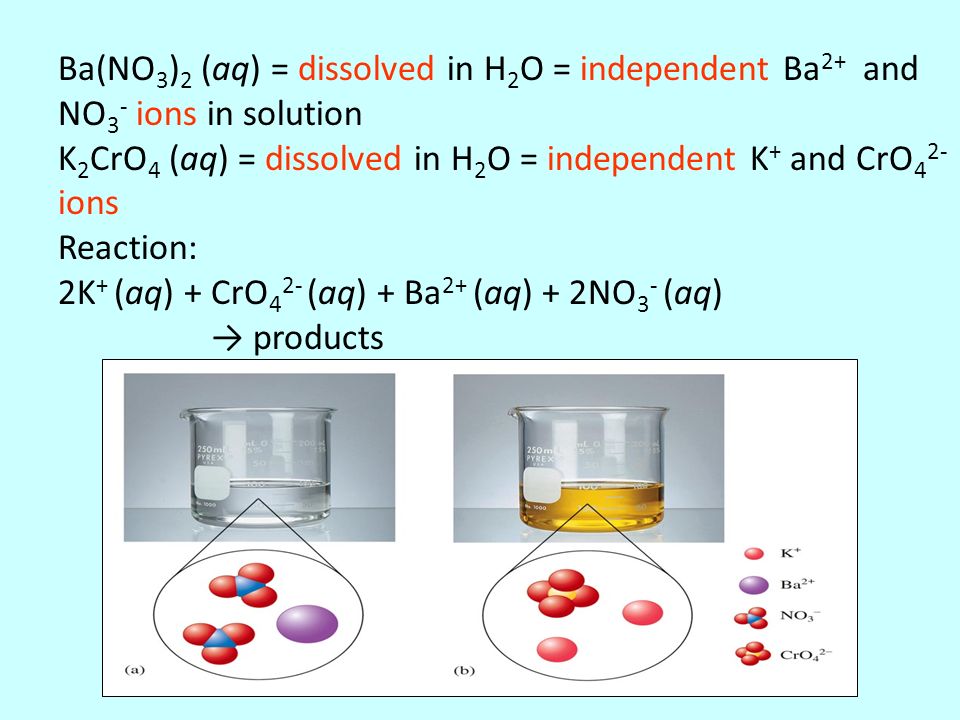
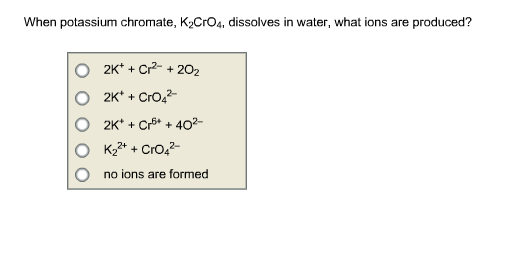
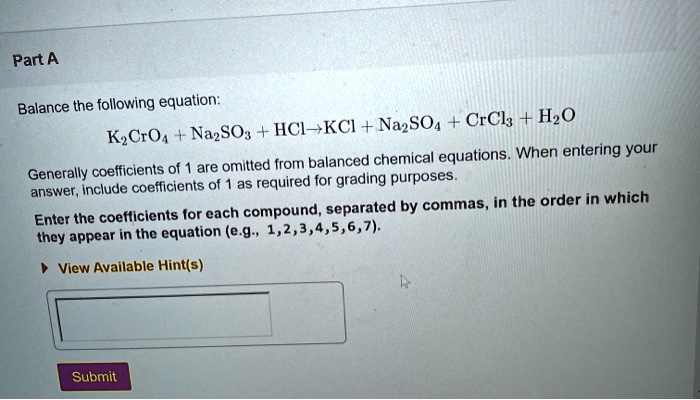
K2CrO4 + K2SO3 + H20 -> Cr(OH)3 + K2SO4+ KOH Uzupełnij współczynniki Bilansem jonowo-elektronowym. - Brainly.pl
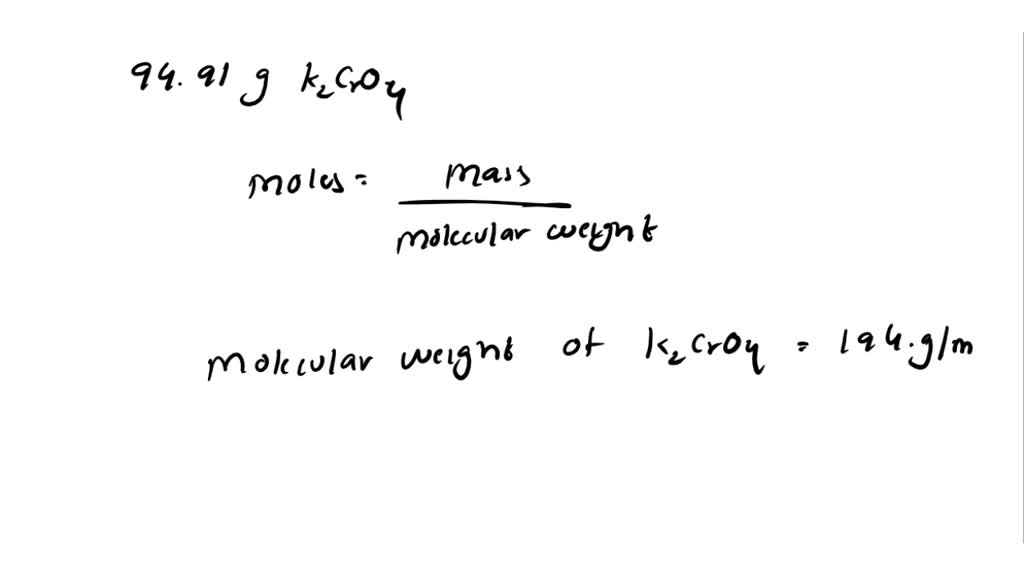
SOLVED: A. How many moles of K2CrO4 are in 94.91 g K2CrO4? B. How many moles of CaC2O4·H2O are in 236.0 mg CaC2O4·H2O?

SOLVED: Potassium chromate (K2CrO4) dissolves in acidic solution to generate strongly oxidizing CrO42- ions: K2CrO4(s) + H3O+(aq) â†' 2K+(aq) + HCrO4-(aq) + H2O(l) 2HCrO4-(aq) + CrO42-(aq) + H2O(l) â†' 3H+(aq) + 2Cr3+(aq) +

SOLVED: A standard aqueous solution of potassium chromate (K2CrO4, MW = 194.2 g/mol) is prepared by dissolving 106.3 g of K2CrO4 in 400.0 g of water (H2O, MW = 18.02 g/mol). The
Q117) The set of numerical coefficients that balances the equation K2CrO4 + HCl → K2Cr2O7 + kCl + H2O is kerala CEE 2001 d) 2, 2, 1, 2, 1
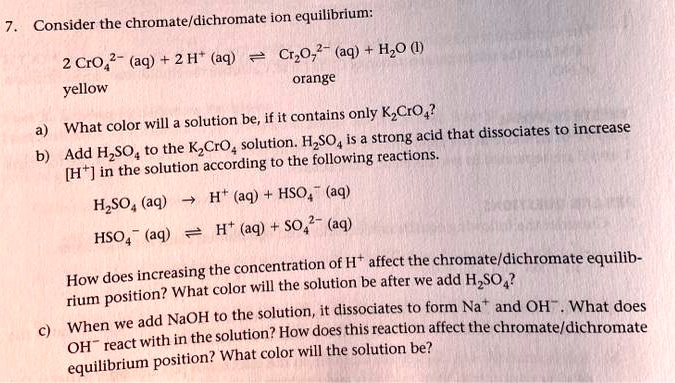
SOLVED: Consider the chromate/dichromate ion equilibrium: CrO4^2- (aq) + H2O (l) ⇌ 2 CrO4^2- (aq) + 2 H+ (aq). The solution will be orange-yellow if it contains only K2CrO4. What color will

MJG Cursillo Química Balanceo de ecuaciones Redox (medio básico): CrI3+Cl2+KOH--K2CrO4+KIO3+KCL+H2O - YouTube