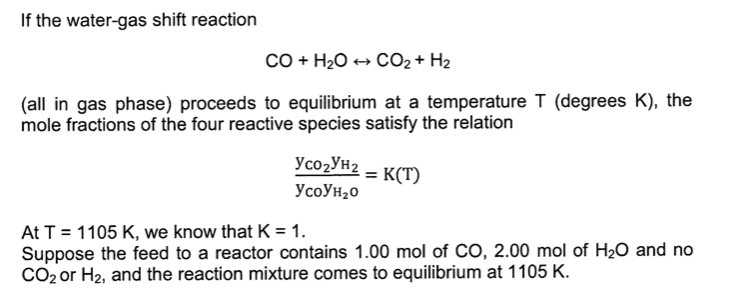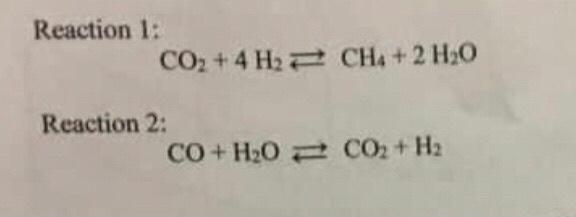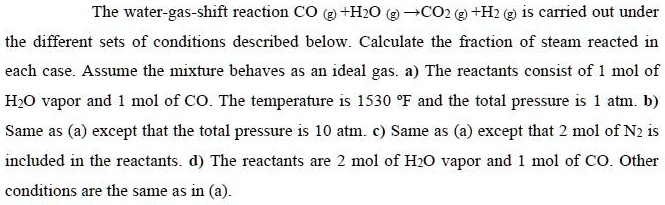
SOLVED: The water-gas-shift reaction CO (g) + H2O (g) â†' CO2 (g) + H2 (g) is carried out under different sets of conditions described below. Calculate the fraction of steam reacted in

I 6.4 The equilibrium constant the reaction, CO + H20 (g) CO2 (g) + H2 (9) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are

CO-ADSORPTION OF CO, H2O AND MECHANISM OF WATER GAS SHIFT REACTION ON ZnO CATALYST SURFACE: A DENSITY FUNCTIONAL THEORY STUDY | Semantic Scholar

Reactions of Photoionization-Induced CO–H2O Cluster: Direct Ab Initio Molecular Dynamics Study | ACS Omega

Radiation-induced synthesis of formic acid in the H2O–CO system: A matrix isolation study - ScienceDirect
An equilibrium mixture, CO(g) + H2O(g) ⇋ CO2 (g) + H2 (g), present in a vessel of one litre capacity at 1000 K - Sarthaks eConnect | Largest Online Education Community

SOLVED: What is the total energy change for the following reaction: CO + H2O -> CO2 + H2? Given: C-O bond: 358 kJ/mol H-O bond: 463 kJ/mol H-H bond: 436 kJ/mol

64 The equilibrium constant the reaction, CO(g) + H2O (9) CO2 (g) + H2 (g) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are placed
Kc for CO(g) +H2O(g) ⇌ CO2(g) +H2(g) at 986°C is 0.63. A mixture of 1 mole H2O(g) - Sarthaks eConnect | Largest Online Education Community



![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/Spectre%20Co6-fi8187013x610.png)


![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/CoH2O6-fi8185737x250.png)