Green Synthesis of 1,4‐Dihydropyridine Derivative in Water - Isomura - 2018 - ChemistrySelect - Wiley Online Library

Mass spectra of RNA 1 (2 μM) in 9:1 H2O/CH3OH with 20 mM CH3COONH4 as... | Download Scientific Diagram
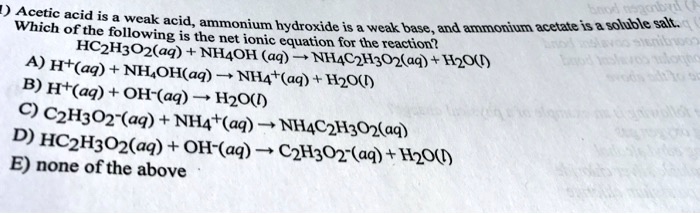
SOLVED: Acetic acid is a weak acid, ammonium hydroxide is a weak base, and ammonium acetate is a soluble salt. The net ionic equation for the reaction is: NH4OH(aq) + HC2H3O2(aq) ->

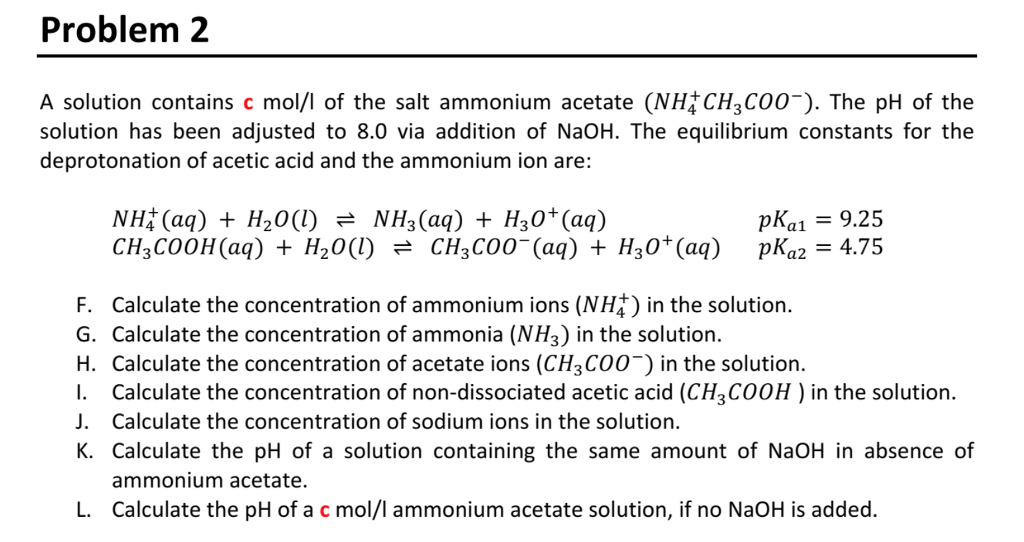
Which of the following has highest value of pH:- 10.1M NaCl 2 0.1M NH4CI 3 0.1M CH3COONa 4 0.1M CH3COONH4
Synthesis of mTPA–PPI ((i) aniline, CH3COONH4, CH3COOH, reflux under N2... | Download Scientific Diagram

Solubility of H2S in (H2O + CH3COONa) and (H2O + CH3COONH4) from 313 to 393 K and at Pressures up to 10 MPa | Journal of Chemical & Engineering Data

Написать молекулярное, полное и сокращенное ионное уравнения реакции NaCl+H20 CH3COONH4+H2O - Школьные Знания.com
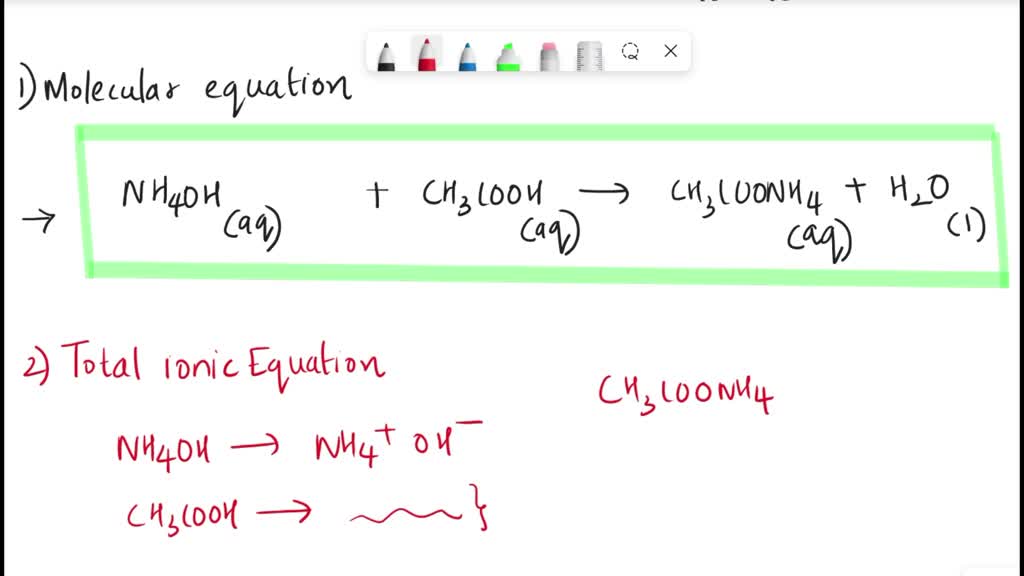
SOLVED: Ammonium hydroxide + acetic acid â†' ammonium acetate + water (a) Molecular Equation (b) Total Ionic Equation (c) Net Ionic Equation with states

Formation of adenine from CH3COONH4/NH4HCO3—the probable prebiotic route for adenine - ScienceDirect

![Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS: Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS:](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjJbWjsn-LBqxX4y74j0W2DM5MJz-FPpzBl0Sn2S3KZpbegpZvPApc4JmvPT4I3cwFInFG4BZE3E4x2cYaQys3MI3C6K0Py8tIkE3hWaKVpxk-jBlgrGQPHBLUZ4XcVBrz1C1IGUAtPybQ/s1600/SH21.PNG)

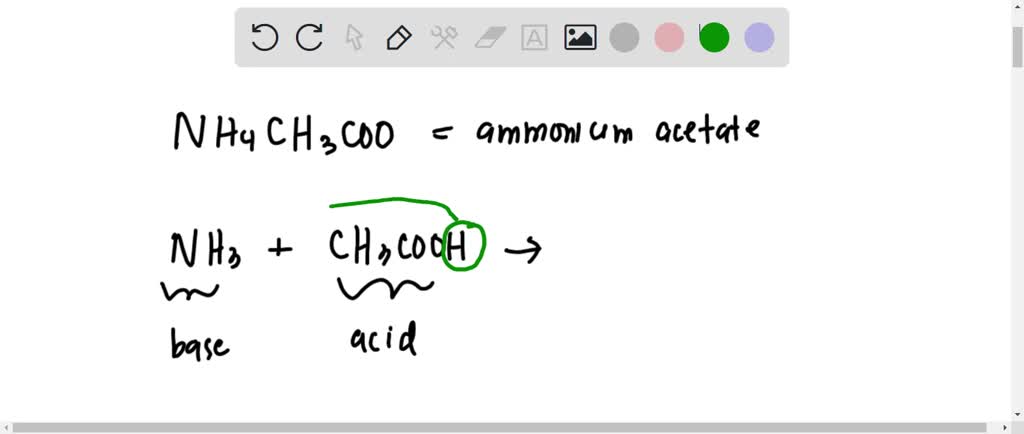
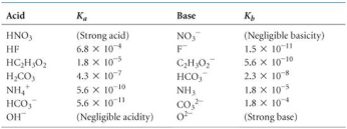
![Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS: Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS:](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgL8SQhnjKci8Sd_t_-hwKS6TbUzdVEN36ZVX7jzyaaImcB0R3-pjiQk5I7lhygJIO2mioVSY0269QkoVgPgOTPfaPDfCu_McwLwOKS5MWlvzzjd4qRRjyHV5vZto0d67HeHHMAvK9k81o/s1600/SH28.PNG)