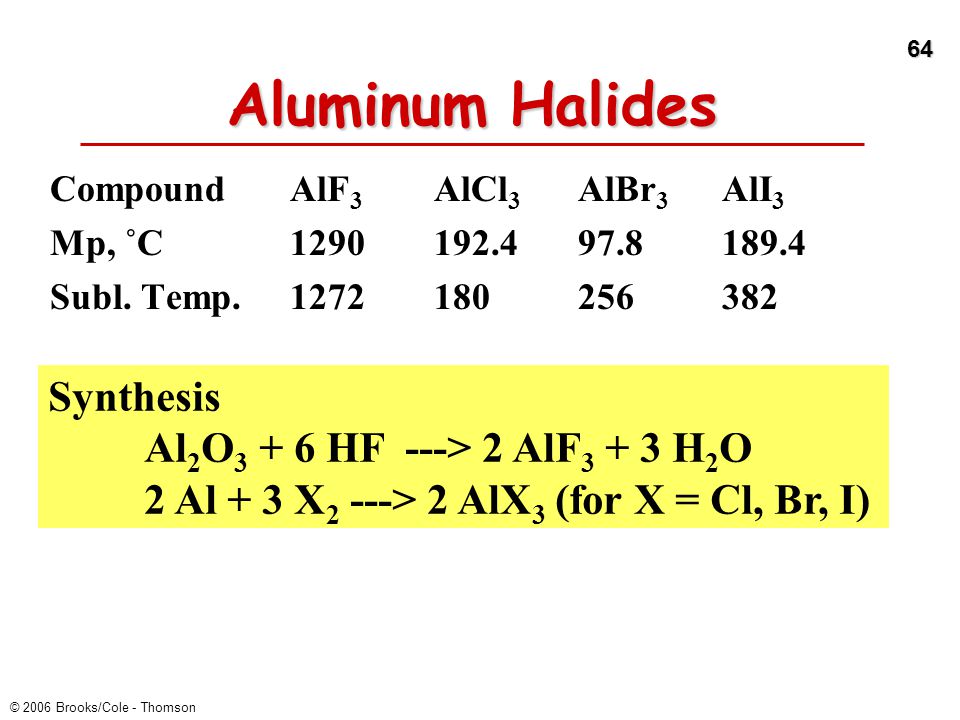
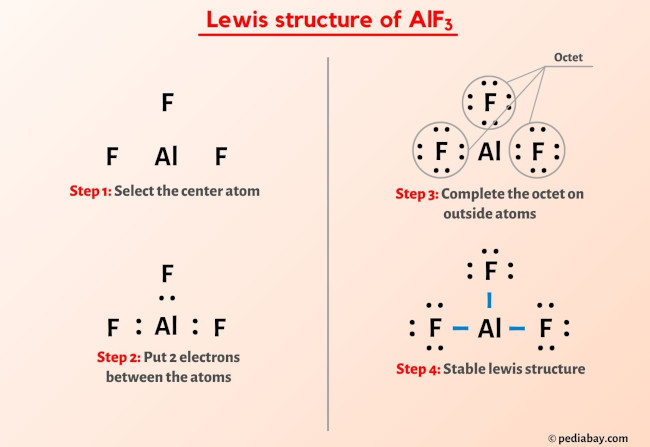
Atomic Layer Etching of AlF3 Using Sequential, Self-Limiting Thermal Reactions with Sn(acac)2 and Hydrogen Fluoride | The Journal of Physical Chemistry C

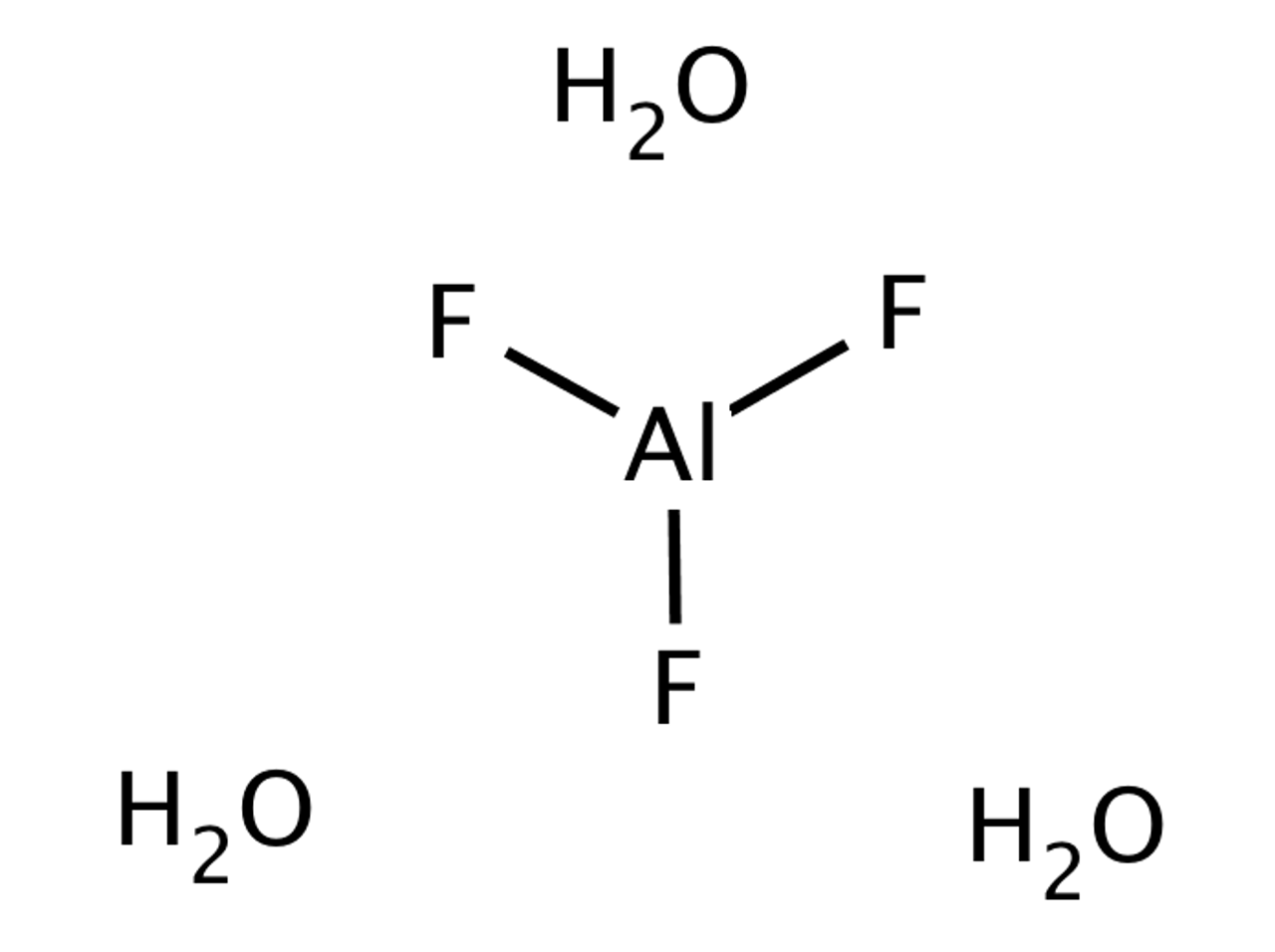
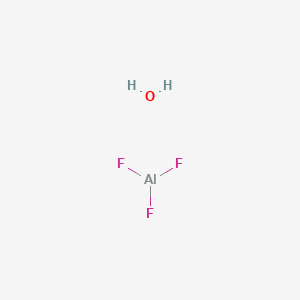
Hydrate de fluorure d'aluminium, Puratronic , 99,99 % (base métallique), Thermo Scientific Chemicals | Fisher Scientific

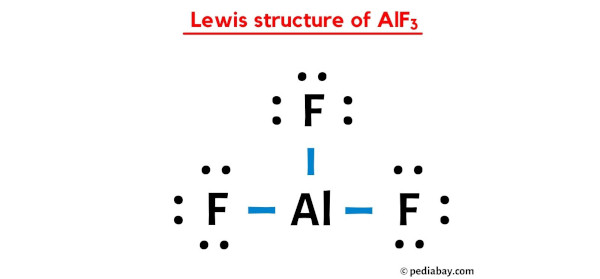
Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar

Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment | ACS Applied Energy Materials
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/4-Figure1-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar
![The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1293255816303363-fx1.jpg)
The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect
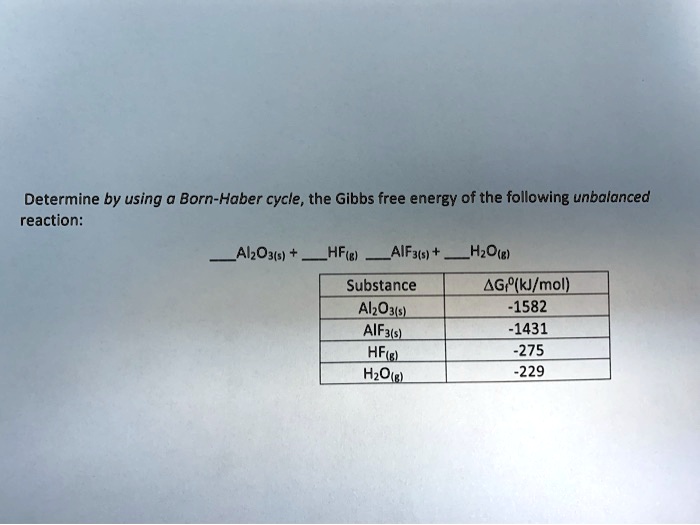
SOLVED: Determine, by using the Born-Haber cycle, the Gibbs free energy of the following unbalanced reaction: Al2O3(s) + 2HF(g) â†' 2AlF3(s) + 3H2O(l) AGr%(kJ/mol) -1582 1431 -275 -229 Substance Al2O3(s) AlF3(s) HF(g)

The isomeric structures of the HClO4/n(AlF3) superacids (for n = 1–2).... | Download Scientific Diagram

The molecular graphs of the AlF3-C2H2 (the top left), AlBr3-C2H4 (the... | Download Scientific Diagram
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/8-Figure4-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar

The acid strength of the datively bound complexes involving AlF3 lone pair acceptor and various lone pair donors - ScienceDirect