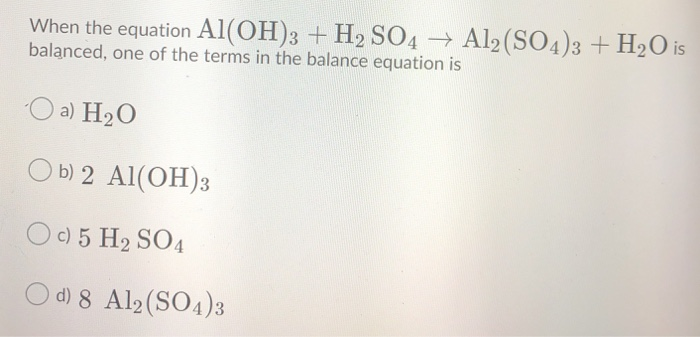OneClass: Mixing 39.0g of Al(OH)3 with an excess of H2SO4 we produce Al2(SO4)3. Using the following e...

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data

Mass and Molar quantities of Ca(OH2) and Al2(SO4)3 · 18 H2O used in the... | Download Scientific Diagram

Consider the reaction of Al2O3 withH2SO4 to form Al2(SO4)3 and H2O. If 3.84 g AL2O3 is reacted with excess - brainly.com

Aluminium sulphate solution 30 % Al2(SO4)3 * 18 H2O for Blue Number determination Contents: 2, 529,55 €

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data

Aluminium sulphate solution 0.2 % Al2(SO4)3 * 18 H2O for blue number determination Contents:, 48,91 €

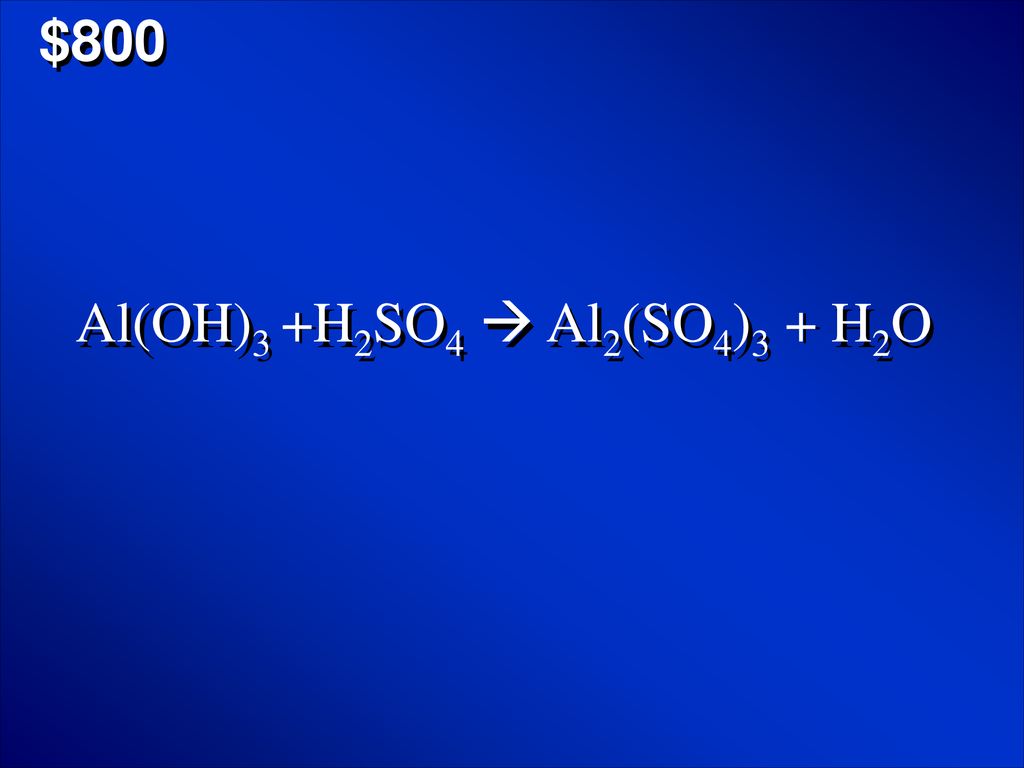
![Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/aluminium-sulfate-octahydrate-molecular-weight-calculation-300x178.jpg)




![Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3 Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3](https://static.doubtnut.com/ss/web-overlay-thumb/10301342.webp)